The landscape of cancer treatment has undergone a monumental shift, moving beyond traditional methods like chemotherapy and radiation to harness the power of the body’s own defense system. At the heart of this revolution is a class of therapies that target what are known as immune checkpoints. These checkpoints are not a flaw in the body’s design; rather, they are a vital safety mechanism, a series of proteins that act as a “brake” on the immune system to prevent it from attacking healthy cells. Cancer, with its remarkable ability to adapt, has learned to exploit these brakes, using them to evade detection and destruction by the immune system’s T-cells. Understanding this intricate biological dance is the key to unlocking new, more effective forms of treatment.
Understanding this intricate biological dance is the key to unlocking new, more effective forms of treatment.
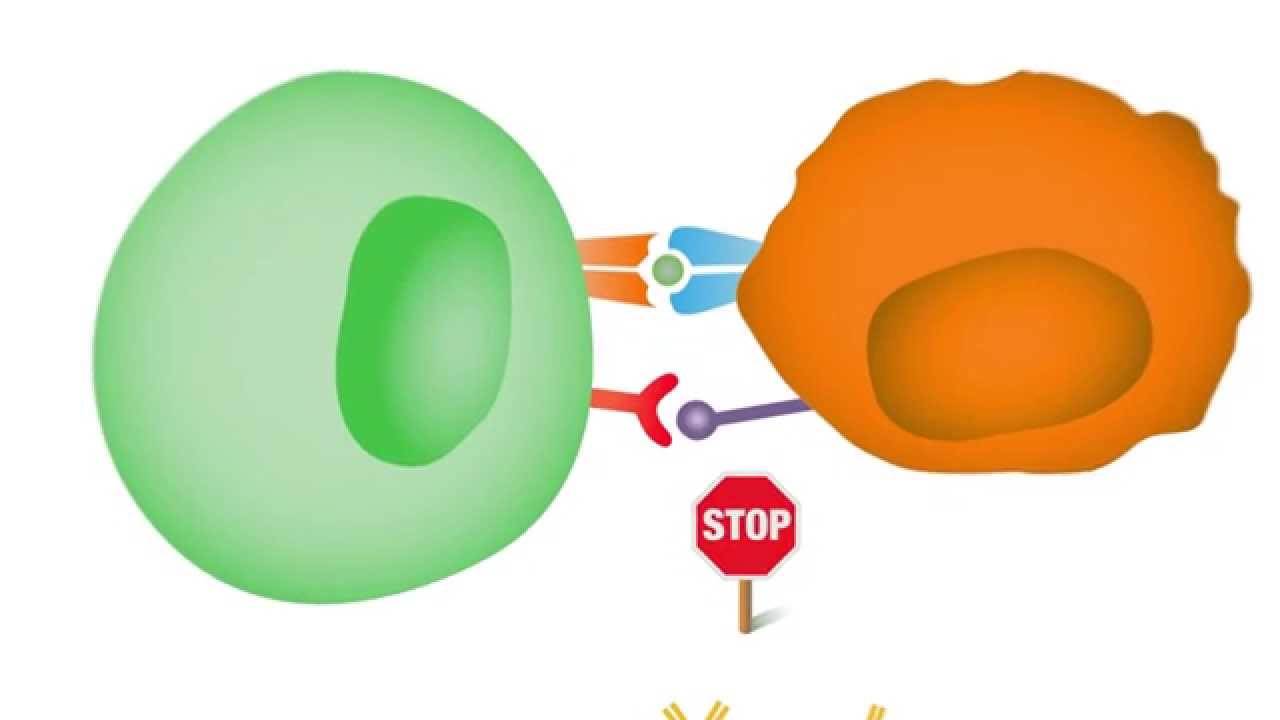
To grasp the concept of immune checkpoints, it’s helpful to think of the immune system as an army. T-cells are the elite soldiers, trained to identify and eliminate threats. To prevent friendly fire, T-cells are equipped with receptors that must interact with specific molecules on the surface of other cells. When a T-cell recognizes a foreign or abnormal cell, it receives a signal to activate and attack. However, healthy cells display certain proteins that act as “don’t attack me” signals, which engage the T-cell’s immune checkpoints, effectively deactivating the T-cell. This delicate balance is what maintains a healthy immune response. Cancer cells, in their cunning, often overexpress these “don’t attack me” proteins, essentially putting a shield up that blinds the T-cells and allows the tumor to grow unchecked.
Cancer cells, in their cunning, often overexpress these “don’t attack me” proteins.
The most studied immune checkpoints are PD-1 and its ligand, PD-L1, and CTLA-4. PD-1 is a protein on the surface of T-cells that, when it binds to PD-L1 on the surface of a cancer cell, sends a signal to the T-cell to stand down. This is one of cancer’s primary escape routes. CTLA-4 is another checkpoint protein, expressed on the surface of T-cells, that plays a role in the initial stages of the immune response, acting as a brake to prevent an overly aggressive attack. By targeting these specific pathways, scientists have developed a new class of drugs known as checkpoint inhibitors. These drugs are a form of immunotherapy that doesn’t attack the cancer directly but rather takes the brakes off the immune system, allowing it to do its job.
By targeting these specific pathways, scientists have developed a new class of drugs known as checkpoint inhibitors.
Checkpoint inhibitors are typically large protein molecules, known as monoclonal antibodies, that are designed to bind to either the immune checkpoint receptor on the T-cell (like PD-1 or CTLA-4) or the ligand on the cancer cell (like PD-L1). By blocking this interaction, the inhibitor essentially removes the shield, leaving the cancer cell vulnerable to attack by the T-cell. The T-cell, no longer receiving the “stand down” signal, is re-engaged and can proceed with its mission to destroy the cancerous cells. This mechanism of action is fundamentally different from chemotherapy, which is a blunt instrument that kills all rapidly dividing cells, healthy and cancerous alike.
The T-cell, no longer receiving the “stand down” signal, is re-engaged and can proceed with its mission to destroy the cancerous cells.
The clinical success of checkpoint inhibitors has been remarkable, particularly in certain types of cancer that were previously difficult to treat. Melanoma, a form of skin cancer, was one of the first cancers to show dramatic responses to these therapies, with patients experiencing long-lasting remission. Since then, checkpoint inhibitors have been approved for a wide range of other cancers, including certain types of lung, bladder, and kidney cancer. The effectiveness of these drugs is often measured not just by tumor shrinkage but by the duration of the response. Many patients who respond to checkpoint inhibitors experience a sustained, durable control of their disease, which is a significant improvement over many older therapies.
Many patients who respond to checkpoint inhibitors experience a sustained, durable control of their disease.
However, checkpoint inhibitor therapy is not a one-size-fits-all solution. Not every patient responds to these drugs, and a significant portion of patients who do respond will eventually develop resistance. This is because cancer is an incredibly adaptive disease, and it can find new ways to evade the immune system. One of the major challenges in the field is identifying biomarkers that can predict which patients are most likely to respond to a given therapy. For example, some tumors with a high number of mutations seem to be more “visible” to the immune system and are more likely to respond to PD-1/PD-L1 inhibitors. The search for these predictive biomarkers is a major area of ongoing research.
One of the major challenges in the field is identifying biomarkers that can predict which patients are most likely to respond to a given therapy.
Another unique aspect of checkpoint inhibitor therapy is the management of side effects. Because these drugs are designed to unleash the immune system, they can sometimes cause the immune system to attack healthy tissues. These are known as immune-related adverse events (irAEs). They can affect almost any organ system in the body, from the skin and digestive tract to the lungs and endocrine system. The side effects are often different from those of chemotherapy, and they require a specific approach to management, often involving a course of steroids to calm the overactive immune response. A patient’s care team must be vigilant in monitoring for these unique side effects.
A patient’s care team must be vigilant in monitoring for these unique side effects.
The future of cancer immunotherapy lies in combining these treatments. Checkpoint inhibitors are now being used in combination with other forms of therapy, including chemotherapy, radiation, and even other immunotherapies. The rationale behind these combinations is to create a more potent and comprehensive attack on the cancer. For instance, radiation therapy can kill cancer cells and release tumor antigens, which can then be picked up by the immune system and presented to T-cells, potentially making the tumor more susceptible to a checkpoint inhibitor. The development of these synergistic therapies represents the next frontier in cancer treatment.
The development of these synergistic therapies represents the next frontier in cancer treatment.
Looking ahead, researchers are exploring new immune checkpoints beyond PD-1 and CTLA-4. There are many other potential “brakes” on the immune system that could be targeted. Additionally, the field is moving towards more personalized medicine, where a patient’s unique tumor characteristics and genetic profile are used to guide treatment decisions. This approach aims to match the right patient with the right therapy, improving efficacy and reducing unnecessary side effects.
The field is moving towards more personalized medicine.
The discovery and clinical application of immune checkpoint inhibitors represent a paradigm shift in oncology. They have transformed the way we think about cancer, moving it from a battle against a rogue cell to a complex negotiation with the body’s own immune system. This new understanding offers hope for patients who previously had few options and promises a future where cancer is not always a life-ending diagnosis.